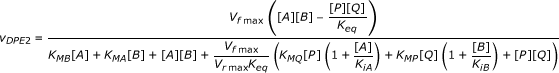
|
Haldane relation 1 | 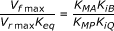 [30] [30]
|
Haldane relation 2 |  [30] [30]
|
K
eq
| 1.0 [53] |
K
MA
4
| 4.6 mM [55] |
K
MB
1
| 1.1 mM [55] |
V
fmax
3
|  ; [DPE2] = 2 μM ; [DPE2] = 2 μM
|
K
MP
3
| 11.7 mM [55] |
K
MQ
1
| 1.1 mM [55] |
K
iB
2
| 1.0 mM [55] |
K
iQ
2
| 1.0 mM [55] |
K
iP
3
| 5.57 mM |
K
iA
5
| 2.19 mM |
- Concentrations A= maltose, B = arabinogalactan, P = glucose, and Q = glucosylated arabinogalactan.
- 1 The K
M
(arabinogalactan) and K
M
(glucosylated arabinogalactan) values were taken from the disproportionation reaction between 4-nitrophenyl-α-D-maltoheptaoside-4-6-O-ethylidene (EPS) as donor and maltotetraose as acceptor catalyzed by the cyclodextrin glycosyltransferase (CGTase) enzyme in Thermoanaerobacterium thermosulfurigenes.
- 2Substituted by the T. thermosulfurigenes K
i, competitive
(γ-cyclodextrin) value for the disproportionation reaction between EPS as donor and maltose as acceptor catalyzed by the T. thermosulfurigenes CGTase.
- 3Taken from the DPE1 model.
- 4 Taken from T. thermosulfurigenes K
M
(maltose) value for the disproportionation reaction between EPS as donor and maltose as acceptor catalyzed by the T. thermosulfurigenes CGTase.
- 5From Haldane relations 1 and 2 and values for associated kinetic constants given.